 |
A little more on Wavelength and Monochromatic Radiation |
 |
A little more on Wavelength and Monochromatic Radiation |
A little more on Wavelength and Monochromatic Radiation
X-rays belong to the short wavelength end of the electromagnetic spectrum. We can think of them as sinusoidal waves of electromagnetic vibration, travelling at the speed of light (see below).
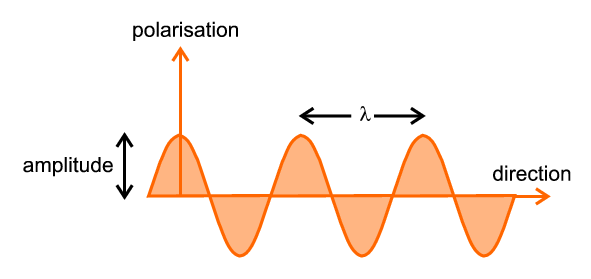
The most important parameter is the wavelength, illustrated and denoted by λ, which is the distance between successive crests (or troughs) of the travelling wave. If a source of radiation produces waves, all with the same wavelength, it is said to be a monochromatic source; alternatively a source producing a wide range of wavelengths is said to be continuous, white, or Brehmsstrahlung.
By far the most common monochromatic radiation used to the present
time has been the characteristic (so-called Kα)
X-rays produced by a laboratory copper X-ray tube source (further details will
be given later in the course). This produces X-rays with a wavelength around
When such rays travel through matter the vibrating electric/magnetic fields
interact with atoms, and are scattered by the constituent atoms. A measure of
the
| E (keV) | ≈ | 12.4 ——— λ (Å) |
Thus the shorter the wavelength, the more energetic the X-ray and the greater its penetrating power.
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Tony Csoka Simon Jacques |