 |
Introduction |
 Course Material Index
Course Material Index
 Section Index
Section Index
 Next Page
Next Page
Introduction
Neutrons are often pictured as tiny sub-atomic particles that make up
approximately 50% of the mass of most atoms.
However, due to the principle of wave-particle duality,
neutrons also have a wavelength.
The momentum, energy, spin, and lack of charge of the neutron makes it
an ideal probe of the structure of matter.
Neutrons were discovered many years after X-rays, and it was only
after the development of nuclear reactors that intense beams of
neutrons became viable for research use.
The environmentally-friendly alternative to nuclear reactors for
the production of intense beams of neutrons is the pulsed spallation source.
Crystal structure refinement from powder data was first developed
for the case of neutron diffraction, resulting in the
Rietveld method, which is still in use today.
This section of the course explains why
neutrons are used, together with powder neutron diffractometer design
and function.
Neutron versus X-ray powder diffraction
Before discussing powder neutron diffraction in detail, a quick comparison
with X-rays may be useful for those less familiar with the use of neutrons
for powder diffraction studies.
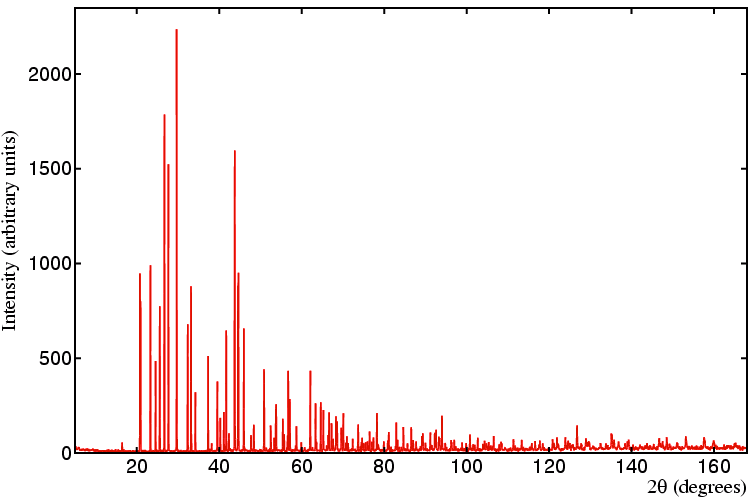
The figure below shows a typical laboratory data set
of the mineral anglesite, PbSO4,
collected with Cu Kα1 radiation
in Bragg-Brentano geometry (to avoid absorption problems due to the
lead atoms).
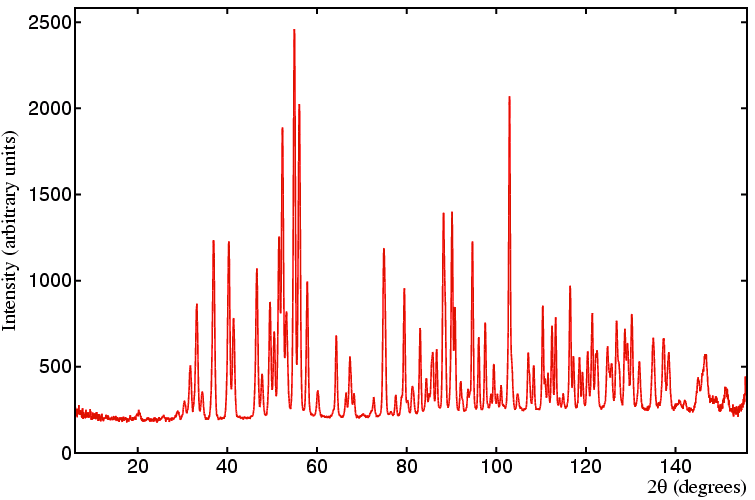
 For comparison, a neutron diffraction pattern is shown of the same material
collected on a constant wavelength medium-resolution powder
diffractometer (D1A at the ILL, Grenoble)
with a neutron wavelength equal to 1.909 Å.
For comparison, a neutron diffraction pattern is shown of the same material
collected on a constant wavelength medium-resolution powder
diffractometer (D1A at the ILL, Grenoble)
with a neutron wavelength equal to 1.909 Å.
 There are several obvious differences:
There are several obvious differences:
- Since the data sets were collected with different wavelengths, peaks due
to identical d spacings will obviously appear at different angles (due
to Bragg's law). (A more direct comparison
can be made by plotting in either
d or 1/d, but there are various arguments both
for and against this that will not be discussed in detail here.)
-
The relative intensities of adjacent peaks are very different in the two data
sets. Reflections that exhibit a high intensity in the X-ray data set may
be relatively weak when measured by neutron diffraction, and vice-versa.
-
For the X-ray data set, the intensity of the reflections decays with increasing
scattering angle due to the effect of the X-ray form factor, f, in stark
constrast to that observed in the neutron data set, where the peaks are still
intense at high scattering angle.
-
For the particular data sets shown, the peaks are a lot broader
in the neutron data set than in the X-ray data set due to
the different instrumental resolution functions. It is probably less
obvious that the X-ray resolution function has its minimum at a relatively
low scattering angle, while the minimum is observed at a relatively high
scattering angle in the neutron case.
-
The neutron data set has a much higher background than the equivalent X-ray
data set, thus giving a worse peak to background ratio.
The remainder of this section will attempt to explain why some of
these differences occur and
how powder neutron diffraction experiments exploit them.
 Course Material Index
Course Material Index
 Section Index
Section Index
 Next Page
Next Page



