 |
Properties of the Neutron |
 |
Properties of the Neutron |
Properties of the Neutron
The neutron is a particle with a spin of one-half. It is the interaction of this spin with the spin state of the nucleus of an atom that determines the scattering property of a neutron with that atom. This spin-spin interaction leads both to coherent scattering, and hence diffraction effects, and to incoherent scattering, which for the purposes of powder neutron diffraction ends up as general background noise in the data. A neutron may also simply be absorbed by the nucleus of an atom, a property that is made use of in the detection of neutrons. Finally, though it goes beyond the scope of this course, the spin of the neutron may interact with the spin of any unpaired electrons of an atoms, which leads to so-called magnetic scattering. The following paragraphs describe these interactions in more detail.
Scattering length b
For the case of X-ray diffraction, the intensity of a reflection, Ihkl, is proportional to the square of its structure factor, Fhkl. As shown in the section on diffraction theory, the latter is given by the equation
| N | ||
| Fhkl = | Σ | |
| n=1 |
where fn is the scattering factor for atom n within the unit cell. For neutron diffraction, the structure factor is given by a similar expression with the single exception that the X-ray scattering factor, f, is replaced by the neutron scattering length, b. The X-ray scattering factor, f, of an atom is quite different to the neutron scattering factor, b, of the same atom. The reason is that X-ray diffraction results from scattering by the electrons of an atom while neutron (non-magnetic) diffraction is due to scattering from the nucleus of an atom. This has two consequences:
Firstly, the atomic nucleus acts a pin-point scatterer unlike the electron cloud, which is of finite size compared to the wavelengths used. The implication of this is that b is independent of the scattering angle, 2θ, in contrast to the X-ray case where f has a form factor that decreases with increasing scattering angle. Thus the smaller d spacing information is relatively better determined in a neutron experiment compared to a similar X-ray experiment. This results in more precise atomic thermal parameters being determined from powder neutron data.
Secondly, for X-rays, f at zero scattering angle, is simply
equal to the number of electrons in the atom (or ion), i.e.
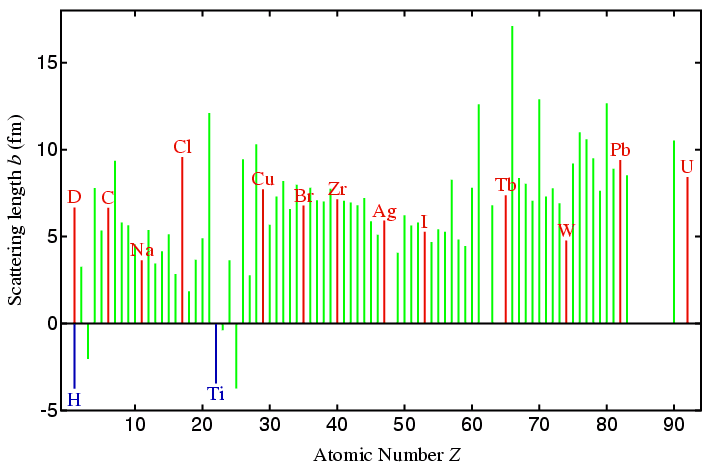
The table that follows lists values for the neutron scattering lengths
of a few atoms together with their atomic number to allow a simple
comparison with the X-ray case. (A direct comparison can be made
by multiplying f (0°) value by the classical electron radius,
re, equal to 2.818 fm. However, X-ray scattering lengths
are rarely used in practice since the proportionality constant
re is best ignored for most practical purposes.)
| Element | H | D | U | Fe | Co | Ba | O | V | Ti | Zr |
| X-ray f (0°) (Z) | 1 | 1 | 92 | 26 | 27 | 56 | 8 | 23 | 22 | 40 |
| Neutron b (fm) | -3.740 | 6.674 | 8.420 | 9.450 | 2.780 | 5.280 | 5.805 | -0.3824 | -3.438 | 5.280 |
You should note that "heavy" elements do not scatter on average very much more than lighter ones (compare D and U in the table). In addition, adjacent elements that would be difficult to distinguish in a laboratory X-ray experiment may scatter very differently, a well-know example being that of Fe and Co (as given in the table). Historically, much powder diffraction research with neutrons has been on oxide systems: a comparison of, say, Ba with O in the above table shows the scattering power of these elements is similar in stark contrast to the X-ray case.
A few elements scatter neutrons very weakly, a notable example of this being vanadium, which has a tiny negative scattering length. This property is exploited in the manufacture of sample holders for powder neutron diffraction (see later page). Finally, for some medium high-pressure studies a metal titanium-zirconium (68%:32%, b = 0.0 fm) alloy has been used, which exploits the fact that titanium is one of the few metals with a large negative scattering length.
Absorption cross-section σabs
A consideration of neutron scattering lengths if not the only factor that should be taken into account when contemplating a powder neutron diffraction experiment. For the X-ray case, the scattering and absorption of X-rays both increase with increasing atomic number Z, though with absorption it may suddenly change due to absorption edges (as shown in the section on X-ray filters). Neutrons have the desirable property that they are only absorbed by a few nuclei as shown on the figure below:
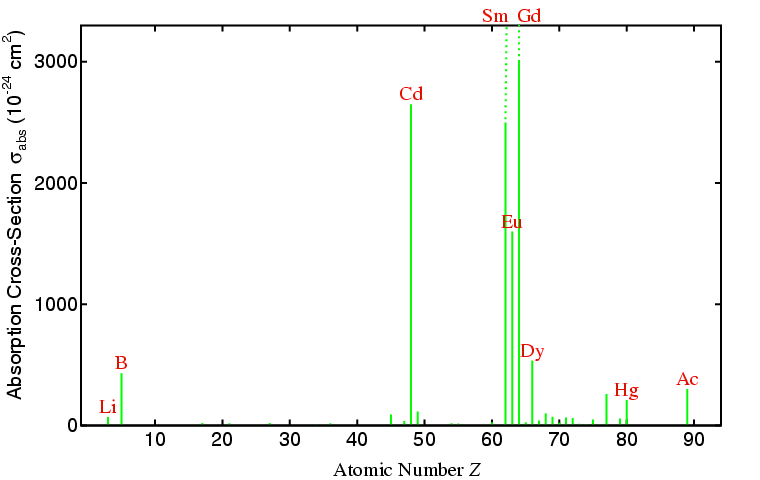
You can see from the above figure that neutron cross-sections (for
absorption and scattering) are very small
numbers when quoted in cm2. They are often given in a
more convenient unit called a barn,
where
Incoherent cross-section σinc
It was mentioned that the spin-spin interaction of the neutron with the nucleus of an atom leads to both coherent and incoherent scattering. How this arises need not concern us. However, you should be aware that a few isotopes have very large cross-sections for incoherent scattering as shown in the following figure:
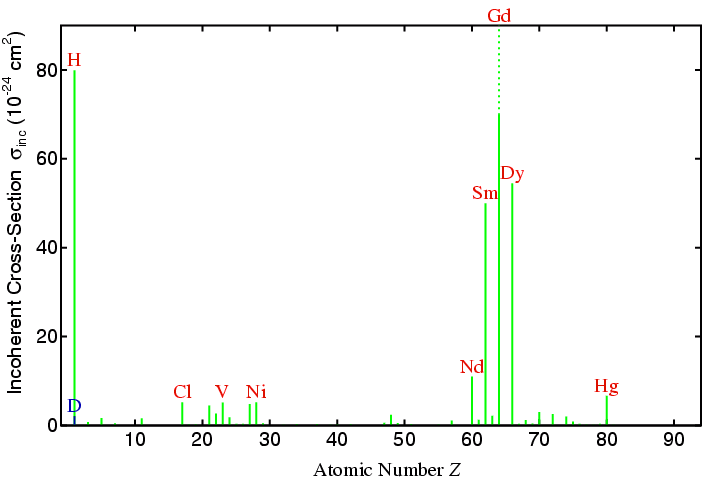
Temperature of the neutron
The kinetic energy, E, of the neutron is given by mv2/ 2, which is conventionally related to temperature via the Boltzmann constant, kB (= 1.381×10-23 JK-1), according to the equation:
Taking room temperature as 20°C, i.e. 293 K, a velocity equal to 2200 ms-1 is obtained for thermal neutrons, which therefore have a wavelength that is approximately equal to 1.8 Å. You may note that many scattering properties of the neutron are quoted for this specific wavelength, velocity, and energy. Neutrons with long wavelengths are often referred to as cold neutrons, while at the other end of the scale, those with short wavelengths are usually known as hot neutrons.
Polarisation
Although neutrons can be polarised in terms of their spin, this property is only important for single-crystal magnetic experiments. For neutron powder diffraction, there is no polarisation factor in contrast to the equivalent the X-ray case.
Generation of neutrons
Intense neutron beams cannot be produced in the laboratory. Currently, there are two methods for producing neutron beams of sufficient intensity for powder diffraction work, namely, a nuclear reactor and a pulsed source. The two methods are quite different and result in neutron beams with different characteristics. Both methods involve fission, though with a pulsed source it is not necessary to use a naturally fissile material. The consequences of the two methods in terms of the design and function of neutron powder diffractometers will be discussed separately in later pages of this section of the course.
| © Copyright 1997-2006. Birkbeck College, University of London. | Author(s): Jeremy Karl Cockcroft |