 |
Screw Symmetry
I. Concepts |
 Course Material Index
Course Material Index
 Section Index
Section Index
 Previous Page
Previous Page
 Next Page
Next Page
Concepts
So far you have seen examples of pure rotational symmetry and
pure translational symmetry.
These two types of symmetry element may be combined together to form
a single symmetry element known as a screw axis.
Before considering this symmetry element in detail, it is instructive
to consider why it occurs in crystal structures in the first place.
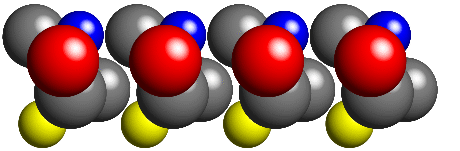
Consider a line of molecules (where each "molecule" consists of 3 grey
spheres, and 1 each of red, blue, and yellow) arranged in a
1-dimensional lattice as shown in the picture below:
A second line of molecules may be packed adjacent to the first
in several ways. If the molecules have the same orientation as the first row,
then the second row will simply form part of a 2-dimensional lattice as
discussed earlier.
Another possibility is that the second row is rotated about an axis parallel
to the first by, for example, 180°, as shown below:
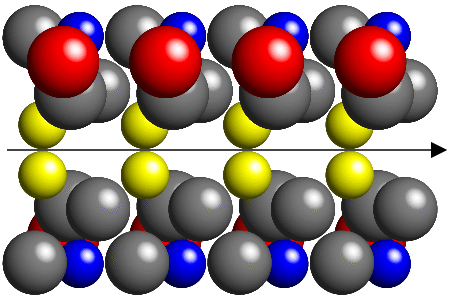
Note that this form of molecular packing tends to leave large holes and,
in general, will be energetically unfavourable within a crystal.
In order for the molecules to be packed closer together, it is necessary to
both rotate and translate the molecules as shown in the next figure:
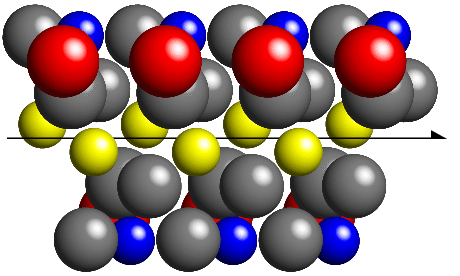
By combining rotation with translation, the molecules can pack closer together
without leaving large holes in the crystal structure. You should be able
to see that the amount of translation involved in the above packing
is exactly half the repeat distance of the molecules
along the axis direction.
This type of symmetry element, called a screw axis, is commonly observed
in molecular crystal structures.
The one shown above, indicated by the line with a half arrow-head,
is called a "two-one" axis
and it has the crystallographic notation 21.
 Course Material Index
Course Material Index
 Section Index
Section Index
 Previous Page
Previous Page
 Next Page
Next Page




