 |
Combined Techniques
II. Energy Dispersive, EXAFS & Neutrons |
 |
Combined Techniques
II. Energy Dispersive, EXAFS & Neutrons |
Energy Dispersive, EXAFS & Neutrons all come together
We will return to the X-ray diffraction analysis but let us first look at the results from, yet, a third technique - neutron scattering/diffraction obtained from instruments on the ILL-Grenoble and ISIS-Rutherford sources (below):
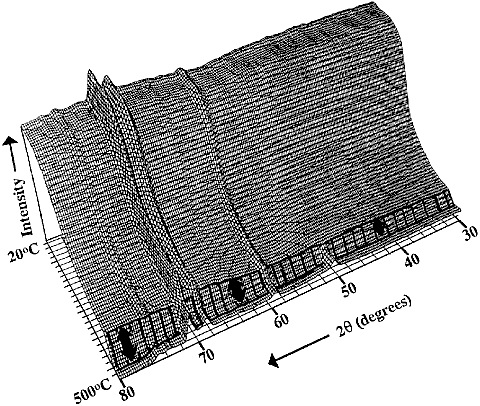
This shows some additional and fascinating information. The high background comes from the hydrogen present as either hydroxide (OH) or water (H2O) units. From the rate of loss of background one can fit rate equations to the data describing the loss of OH/water. However immediately prior to crystallisation there is a dramatic "cliff edge" loss of background - this cliff edge has been highlighted and arrowed in the figure. The cause of this sudden edge-drop was elucidated using difference techniques (see earlier section on the value of neutrons) in which some or all the OH−/H2O was replaced by combinations of OD−/D2O and CH3O−/CH3OH. Since D scatters much more coherently than H, the variation in background could be interpreted in relation to the full/partial substitutions made, in a similar spirit to the earlier example on cobalt acetate (Grimes and Fitch). The conclusion from all of this analysis was that this last moment cliff edge drop in background represented the last vestiges of hydroxides bridges between the zirconium atoms being reduced to oxygen bridges in a cooperative (simultaneous) last push towards crystallisation. The rafts (elucidated by EXAFS on the previous page) had now become "oxide rafts" and were ready to condense together as stacks of cards and three-dimensional crystallisation was assured. To follow the progress from there on one turns again to X-ray diffraction to capture the subsequent behaviour (below):
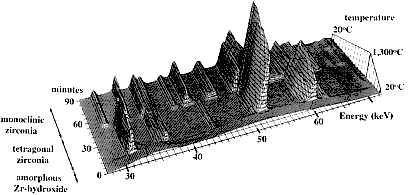 |
|
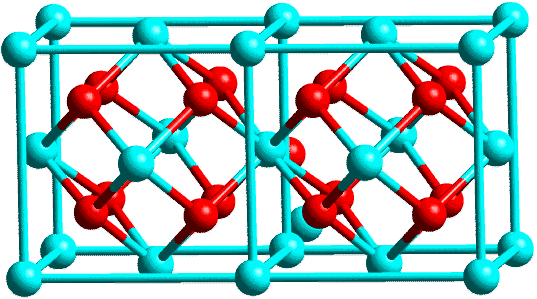
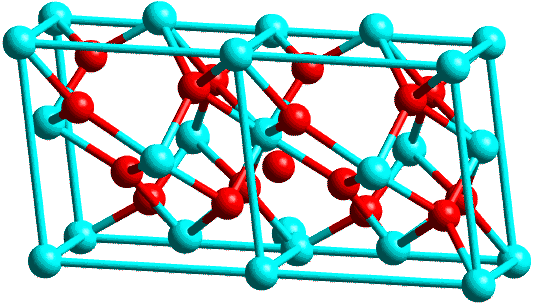
This last figure has been used previously on the course. It shows rather beautifully the overall course of events: the crystallisation as the temperature is raised to 500°C, the continuation of this to the top temperature (in this case 1300°C) and the clear structural transformation from the tetragonal to monoclinic form on cooling. The structures of these two forms are illustrated in the next figure, using two unit cells of each. In the tetragonal form the Zr is surrounded by 8 oxygens whereas it is surrounded by only 7 oxygens in the monoclinic form; the former is a metastable structure whereas the latter is stable.
| Tetragonal: |
 |
| Monoclinic: |
 |
This transformation, and the temperature at which it occurs or would occur, is a vital parameter in the performance of the final zirconia oxide. Its value can range from as high as 1000°C to below room temperature, and this has been measured, in this way, as a function of process engineering variables such as heating rate, top temperature, time at top temperature, cooling rate and pH of the precursor hydroxide. We can now see that to get a total view of the synthesis process it was necessary to assemble various jig-saw like pieces of information provided by each component technique; any one technique alone would have given a very incomplete picture, yet the combined (and mostly simultaneous) techniques allowed the timing of these events to be registered against each other.
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Simon Jacques Martin Vickers |