 |
Combined Techniques
I. Energy Dispersive & EXAFS |
 |
Combined Techniques
I. Energy Dispersive & EXAFS |
Energy Dispersive Diffraction & EXAFS
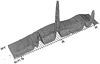
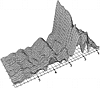
Concentrating on the first stage, the crystallisation of the tetragonal oxide around 500°C for a given batch of material was first studied using a combination of diffraction and EXAFS measurements on the Daresbury UK-SRS synchrotron. Two such time-resolved sequences obtained, involving energy-dispersive diffraction and zirconium-edge EXAFS are shown below:
|
Click image to
enlarge (90 kB) |
A full explanation of these techniques is outside the scope of this course; just the essentials will be explained. The EXAFS patterns involve a Fourier transform of the small variations in X-ray absorption at energies close to those where zirconium absorbs at the K-level. What this tells you is where atoms are, in distance, in the immediate neighbourhood (i.e. the closest 5 Å) of the zirconium atoms. It can do this even though the hydroxide is amorphous. So whereas the early parts of the diffraction pattern look featureless before 500°C at which temperature crystallisation occurs (as evident from the emerging zirconia peaks) the EXAFS peaks can be interpreted. The key information that emerges is that the EXAFS patterns show significant changes from around 450°C. This tells us there is some structural re-arrangement going on some 50°C in advance of crystallisation. However in the spirit of the above philosophy it now falls to re-do the diffraction and EXAFS, but separately in greater detail. To cut a long story short (Turrillas et al. Radiation Phys.& Chem. 45, 491 (1995)) the interpretation of high resolution EXAFS data, combined with other literature, strongly suggested that the hydroxide consists of small rafts with the zirconium atoms (or zirconium cations to be more precise) sitting in approximate square positions with hydroxide or oxygen bridges between and a proportion of water molecules: i.e. it is really a mixed hydroxide/oxide/hydrate as illustrated below:
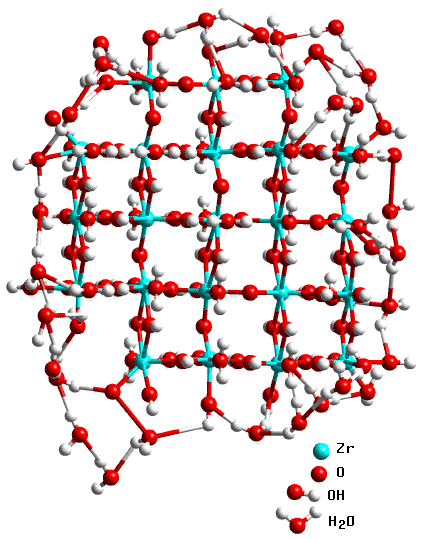
These rafts act as nuclei from which the oxide will eventually crystallise. However it turned out that the crystallisation process itself had some peculiar features that could only be fully interpreted by the use of neutron scattering; this aspect is explained on the next page.
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Simon Jacques Martin Vickers |