 |
The Value of Neutrons
III. Rapid Complex Events |
 |
The Value of Neutrons
III. Rapid Complex Events |
Studying Rapid Complex Chemical Events Using the D20-ILL Instrument
The D20 instrument on the ILL nuclear reactor neutron source has been designed to handle a wide range of relatively rapid (for neutron diffraction) chemical reactions (an equivalent set up, known as GEM, is now also working very successfully on the UK ISIS-spallation neutron source). Two particular features are the closeness of the instrument to the reactor centre, resulting in a very high flux of neutrons on the sample (around 6 to 7×107 neutrons per second per square centimetre of sample cross section), and the remarkable 160° detector which was discussed earlier in a previous section. Click on the image below to see the overall layout of the instrument (kindly supplied by Dr.T.Hansen of the ILL):
 |
104 kB |
This instrument/detector combination system enables the collection of sub-minute diffraction patterns. The layout of the instrument facilitates the installation of special specimen environmental cells which can be craned down into position. Two, of many, examples illustrating the use of this station are given:
(i) The crystallisation of new metal alloys by Dr.S.Kilcoyne et al. (University of St. Andrews, UK). Here the interest was in the crystallisation of amorphous metals which have been formed by rapid quenching of melts onto a spinning copper wheel. One such metal was then subsequently heated on instrument D20 producing the following, somewhat beautiful, sequence (below):
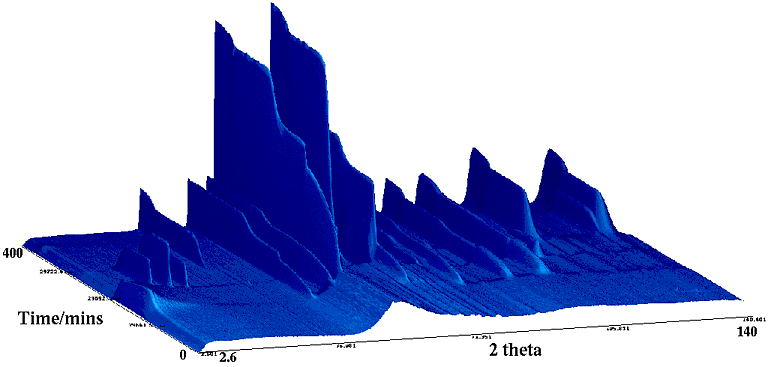
It is easy to see that the time/temperature progression is of an initially amorphous metal (absence of any peaks in the initial patterns) with a known composition of Y2Fe3. It is also easy to work out from the data that crystallisation commences in part around 300°C and is completed by 500°C producing the known cubic YFe2 phase. The surprising feature is the appearance of another phase coexisting with main crystallisation event (note the three transitory peaks at low angles and elsewhere) at around 200-300 minutes (between 390 and 450°C). These peaks were identified as belonging to YFe which is unstable on its own under most temperature conditions, including room temperature, and this explains why its presence had been missed in previous studies.
(ii) Repeated Charge/discharge of LaNi5 electrode systems by M. Latroche and M. Chabre. The interest here is in the electro-chemistry of negative LaNi5 electrodes in equilibrium with the hydride. The charge/discharge process corresponds to the reaction:

From these patterns one can determine the degree of alternation between these states and the associated kinetics using working electrodes similar to those of commercial batteries.
These two examples serve to show the way complex chemical experiments may by exposed to neutron beams for in-situ diffraction experiments. There are endless more examples in the literature and these are but a very small selection.
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Simon Jacques Martin Vickers |