 |
Choice of X-ray Target |
 |
Choice of X-ray Target |
Choice of X-ray Target
 The wavelength, λ, of the characteristic
line giving rise to a particular transition is given by Moseley's Law:
The wavelength, λ, of the characteristic
line giving rise to a particular transition is given by Moseley's Law:
Since the target has to be metallic (so that it conducts electrons) and has to have a reasonably high melting point (40 kV at 30 mA generates 1.2kW of heat), this limits the choice of anode material to chromium (Cr), iron (Fe), cobalt (Co), copper (Cu), molybdenum (Mo), and a few other less commonly used materials for X-ray powder diffraction. The table below shows the Kα radiation for each element:
| Anode | Cr | Fe | Co | Cu | Mo | Ag |
|---|---|---|---|---|---|---|
| Kα (Å) | 2.29 | 1.94 | 1.79 | 1.54 | 0.71 | 0.56 |
Note the big gap in the values between Cu and Mo and the fact that only discreet wavelengths are available in the laboratory. Extensive lists of characteristic lines are given in the International Tables for Crystallography published by the IUCr.
 In many cases, a choice of anode simply doesn't exist, either due to
cost or more likely due to the loss of diffractometer time which
occurs each time the tube is changed. Copper anodes are by far the most
common (as shown above left) since copper
gives the shortest wavelength above 1 Å. The wavelengths
provided by, say, molybdenum and silver are normally too short
for most powder diffraction
work in the laboratory. Short wavelengths both scatter weakly and
contract the diffraction pattern towards low Bragg angles with
consequent loss of d spacing accuracy and resolution.
In many cases, a choice of anode simply doesn't exist, either due to
cost or more likely due to the loss of diffractometer time which
occurs each time the tube is changed. Copper anodes are by far the most
common (as shown above left) since copper
gives the shortest wavelength above 1 Å. The wavelengths
provided by, say, molybdenum and silver are normally too short
for most powder diffraction
work in the laboratory. Short wavelengths both scatter weakly and
contract the diffraction pattern towards low Bragg angles with
consequent loss of d spacing accuracy and resolution.
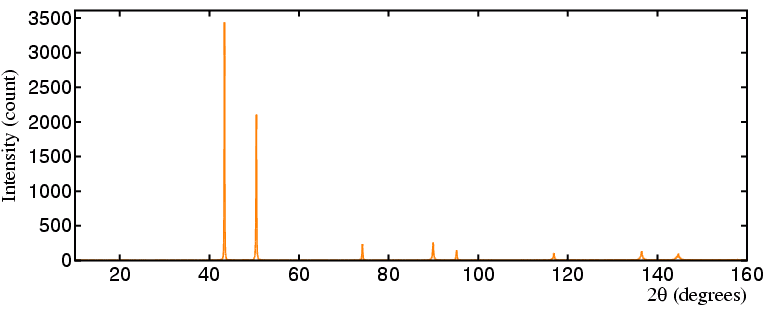
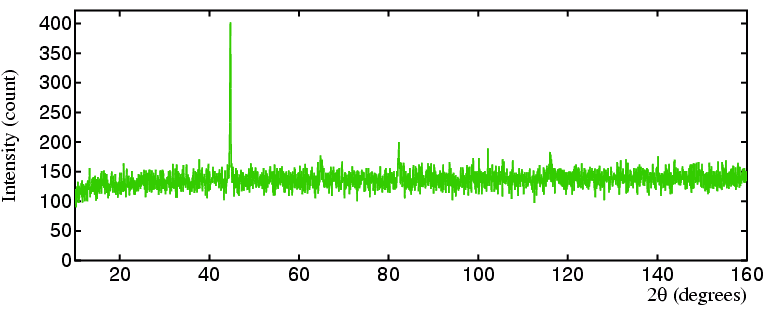
However, for certain research studies copper anodes are definitely not the
best choice as illustrated by the data that follows.
The picture of the coin shows a modern (1998) English "copper" penny from
which the powder diffraction below (in orange)
was measured using a copper anode:
| © Copyright 1997-2006. Birkbeck College, University of London. | Author(s): Jeremy Karl Cockcroft |