 |
Introduction to Space Groups |
 |
Introduction to Space Groups |
Introduction
In order to describe fully the crystal structure of a particular substance, a knowledge of the contents of the unit cell is required. The translational symmetry of the lattice then repeats this information ad infinitum throughout the crystal. However, the unit cell may also contain additional symmetry elements, other than simple lattice translations, such as rotation or screw axes. Under these circumstances, it is not necessary to describe the whole unit cell, but only one part of it. The additional symmetry elements within the unit cell then determine the remainder of its contents. This is illustrated by the following figure showing a single unit cell of the substance sulphur hexafluoride, SF6, at low temperature:
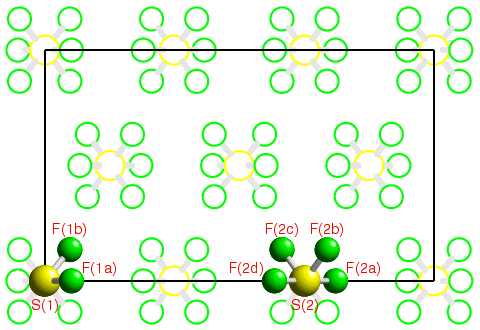
Space-group symmetry is a combination of the translational symmetry of a lattice together with other symmetry elements such as rotation and/or screw axes. The determination of space-group symmetry of material is an essential step in structure analysis since it minimises the amount of information needed for the complete description of the contents of the unit cell. By describing a few of the most commonly-observed space groups in detail, this section on space-group symmetry attempts to cover most of the pertinent concepts of space-group symmetry required in this course.
![]() In the previous section on point-group symmetry used, the symmetry of
an isolated molecule of sulphur hexafluoride was given as
m-3m. In this example, there are two such molecules in the
unit cell, neither of which has symmetry m-3m due to
the presence of neighbouring molecules within the crystal.
Which one of the two molecules labelled S(1) or S(2) do you think has
the highest point-group symmetry in the crystal structure, and why?
(You will get the answer in the next few pages, but try to work out the answer
before reading on.)
In the previous section on point-group symmetry used, the symmetry of
an isolated molecule of sulphur hexafluoride was given as
m-3m. In this example, there are two such molecules in the
unit cell, neither of which has symmetry m-3m due to
the presence of neighbouring molecules within the crystal.
Which one of the two molecules labelled S(1) or S(2) do you think has
the highest point-group symmetry in the crystal structure, and why?
(You will get the answer in the next few pages, but try to work out the answer
before reading on.)
| © Copyright 1995-2006. Birkbeck College, University of London. | Author(s): Jeremy Karl Cockcroft |