 |
 |
 |
| View down 4 axis | Oblique view | Side view |
 |
 |
 |
| View down 4 axis | Oblique view | Side view |
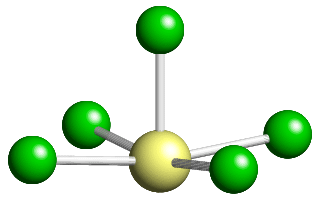
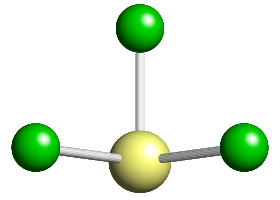
This pentavalent anion can be thought of as a distorted octahedron with one corner atom missing, the corner position being occupied by a lone pair of electrons. The anion has a single fourfold axis, plus 4 mirror planes that intersect along the line of the fourfold axis.
The point-group symmetry for this molecule is given the crystallographic symbol 4 m m.
| © Copyright 1997-2006. Birkbeck College, University of London. | Author(s): Jeremy Karl Cockcroft |