 |
Introduction |
 |
Introduction |
Introduction
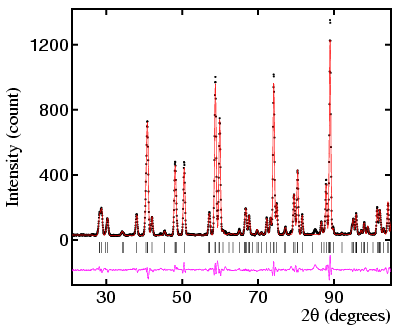
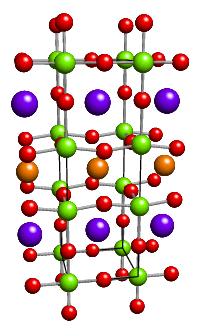
The subject of symmetry plays an essential part in all aspects of crystallography. For the diffraction of X-rays to take place, crystals need to possess some kind of translational symmetry. Inorganic ions, e.g. the Na+ and Cl- in rock salt, have a very high symmetry being spherical. By contrast, organic molecules often have little intrinisic symmetry but nonetheless pack with screw and glide plane symmetries. Although the points just mentioned are only a few examples of where symmetry is found in crystallography, the main need for a thorough understanding of the subject of symmetry is illustrated by the figures below:
|
|
|
A detailed knowledge of symmetry is not required for all aspects of powder diffraction. However, knowledge of the implications of symmetry is probably useful for most aspects of powder diffraction, e.g. to understand why some phases have only a few peaks in qualitative analysis or why one choice of unit cell is preferred to another when indexing a powder diffraction pattern. Clearly, in order to refine a crystal structure from powder diffraction data, then a good understanding of the subject will be required.
This section on symmetry attempts to put the subject on a sound footing so that its use, for example, in indexing, structure solution, and structure refinement is not a mystery to those involved in powder diffractometry.
| © Copyright 1995-2006. Birkbeck College, University of London. | Author(s): Jeremy Karl Cockcroft |