 |
Absorption
I. The Absorption Problem |
 |
Absorption
I. The Absorption Problem |
The Absorption Problem
You may have felt that the approaches so far have made heavy weather of quantitative analysis. One might ask: Why can't the method be as simple as measuring the intensity of diagnostic peaks of each phase in the mixture, measuring the same peaks again from pure standards under identical conditions, then divide the former by the latter? The problem is that in practice the attainment of "identical conditions" for several data sets is quite elusive. Even if one uses Bragg-Brentano geometry, in which the X-ray absorption factor does not change with 2θ-angle, the overall absorbing power of the mixture will be different from those of the pure phases. Worse still, since the composition of the mixture is a priori unknown, the absorption cannot even be calculated. This is why the methods described earlier invariably involved manipulation of ratios of peak intensities within a pattern since these would be from one sample and one measurement and one absorption factor which therefore cancels out on division. There can be no doubting the importance of absorption, since it figures (as parameter A) directly in our basic equation for quantitative analysis:
| Ihkl = (wi / ρi ) c jPLA Fhkl2 |
| wA in mix = |
I(hkl)A in mix
I(hkl) pure A |
The figure below shows these ratios for each mixture plotted against the true weight percentages (X axis). The error bars shown are derived from the esd's on the intensities (peak areas) obtained by peak-fitting; these error bars seem to be consistent with the spread of data points around the two smooth curves, suggesting typical errors of a few weight percent. However, one should always note that there can also be unknown systematic errors and that the example presented here is an ideal case. Real practical problems might not always be so "ideal".
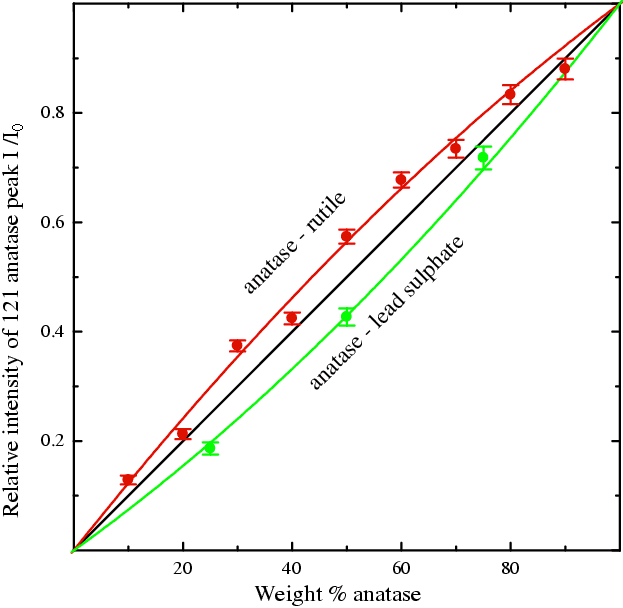
If the absorptions were equal the plot would be a straight line at 45° through the origin (black line). However, it is apparent in both cases that the assumption does not work. Anatase is about 10% less dense than rutile and even though this is a small difference, it seems to be sufficient to cause the plot to deviate from the straight line (compare the red curve and black line), giving an over-estimate of the weight fraction of the anatase. A deviation is also obtained with the anatase + PbSO4, but in the other direction (green line). What is surprising here is that although the absorption contrast between anatase and lead sulphate (1/μ values of 20 and 9 μm respectively) is much greater than between anatase and rutile (20 and 18 μm respectively), the two plots deviate by similar amounts. This tempts one to suppose that factors other than simple absorption are involved: the most likely candidate is micro-absorption. This is related to grain size and is discussed more fully on the next page.
1 L.Alexander and H.Klug, Anal. Chem. 20, 886 (1948)
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Martin Vickers |