 |
Impurity Analysis (Quantitative)
I. Concentration Limits & Impurities |
 |
Impurity Analysis (Quantitative)
I. Concentration Limits & Impurities |
Concentration Limits & Impurities
The previous pages have discussed the commercial importance of new phases, plus the indentification of known phases from small amounts of material. Many materials with industrial application are mixtures, and patent specifications will reflect this in terms of concentration limits and ranges with respect to either discrete phases or solid solutions. The use of powder diffraction for quantitative analysis has already been described; the remainder of this section is concerned with the quantitative analysis of impurities. This raises the question: what factors determine the limits of detection of crystalline phases in powder diffraction?
One of the best methods for testing the lower limits of detection of a discrete phase X in a mixture of phases Y is to collect a diffraction pattern of Y before and after "spiking" it with a small known amount of phase X (see earlier section). For this method to work, the diffraction pattern of phase X must be well known so that diffractometer time can be spent in scanning one or more of the strongest peaks of phase X. It is also important that the peak investigated is due solely to the spiking phase X, i.e. there is no intensity at a similar scattering angle due to other phases in the mixture Y. If there is any possibility of peak intensity due to other phases, then the spiking method will give only an upper limit on the amount of phase X present in the mixture Y.
The figure below shows the diffraction profile (in red) of corundum spiked with sodium chloride measured in the region of the NaCl 200 reflection. The green curve is the scaled diffraction pattern of pure NaCl. In this instance, 0.1% NaCl in Al2O3 is readily detectable.
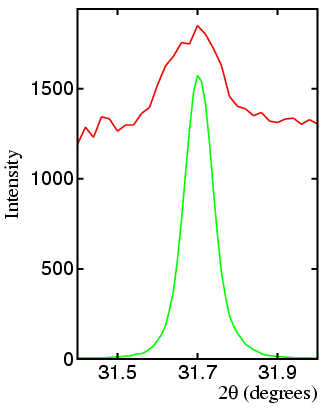
In this instance, as in most cases, the limits of detection are effectively determined by the background count from the sample itself: although the background count will appear close to zero compared to the intensity of the aluminium oxide peaks, it will seem very high with respect to the sodium chloride peak.
In the case study that follows, the judge posed an interesting question:
suppose the court room was full of chalk (i.e. calcite), could one then detect
a spoonful of salt (i.e. sodium chloride) evenly dispersed in it? The answer is
obviously no! (Assuming a court room of size
The next page discusses a patent litigation case in which the possible absence or presence of sodium chloride as in impurity formed a vital part of the trial.
| © Copyright 1997-2006. Birkbeck College, University of London. | Author(s): Jeremy Karl Cockcroft |