 |
Hydrothermal Synthesis
II. Energy Dispersive |
 |
Hydrothermal Synthesis
II. Energy Dispersive |
Energy Dispersive
Hydrothermal syntheses can also be conveniently followed using energy-dispersive (powder) diffraction (EDD) methods, and as pointed out previously this was, historically, the first method used with X-rays. The relative advantages and disadvantages of the EDD and angle-dispersive modes have been mentioned several times in this course, including the high pressure pages given previously. In the context of hydrothermal synthesis the hit list is:
(1) advantages of EDD for hydrothermal synthesis
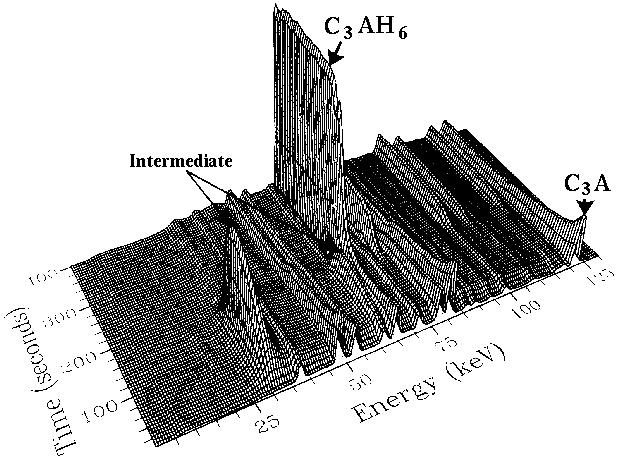
It is now believed that this intermediate phase acts as a nucleating agent for the C3AH6 to start forming, and its crucial role was not realised by the traditional cement community due to the insufficient time-resolution and spatial precision of conventional powder diffraction systems. It serves as a good example of the power of in-situ methods. A related example concerns the setting of oil well cements under autoclave conditions (130 - 250°C). In oilwell operations cement is pumped into well bores for many miles passing through gaps as small as one inch (25 mm). However under the prevailing geothermal conditions of high temperature/pressure the cement can set prematurely, thus blocking the cement flow at a cost that can easily rise into the multi-million dollars range. For the first time the setting of cement within bore-hole autoclave conditions has now been studied (J. Syncrotron Radiation 5, 112 (1998) and 7, 167 (2000)) using energy-dispersive diffraction on the Daresbury SRS synchrotron (below).
 |
Click icon to
expand image (61 kB) |
From this kind of data one is able to obtain phase-concentration plots during the process (below)
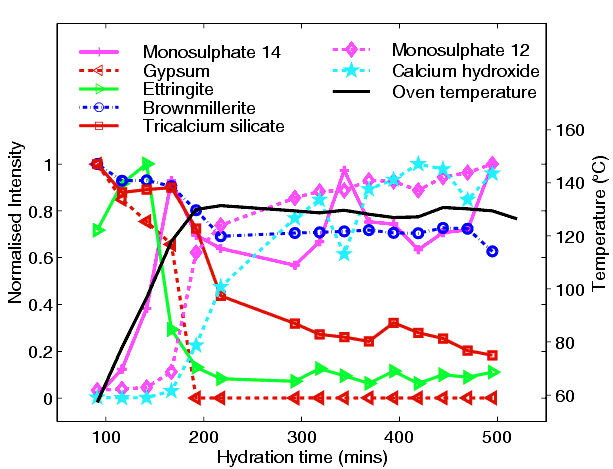
which charts the changes with time, displaying for example a complex interplay between three calcium alumino-sulphate hydrate phases (so-called ettringite, C3A.3Cs.32H; 14-water monosulphate, C3A.Cs.14H; 12-water monosulphate, C3A.Cs.12H; where s = SO3) and the rapid consumption of one phase (known as brownmillerite) which under normal ambient temperature would remain unaffected. So just one successful in-situ experiment has removed all the guesswork and elucidated the true course of hydration under these conditions.
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Sally Colston Simon Jacques Andrew Jupe Martin Vickers |