 |
The Value of Neutrons
I. Catalyst Reduction |
 |
The Value of Neutrons
I. Catalyst Reduction |
Reduction of the Catalyst Molybdenum Trioxide to Molybdenum Dioxide
Molybdenum trioxide, MoO3, is a useful catalyst because of its ability to release its lattice oxygen for the selective oxidation of hydrocarbons; another important application concerns the industrial extraction of metallic molybdenum starting with the reduction of MoO3 to MoO2 with hydrogen at 600°C:
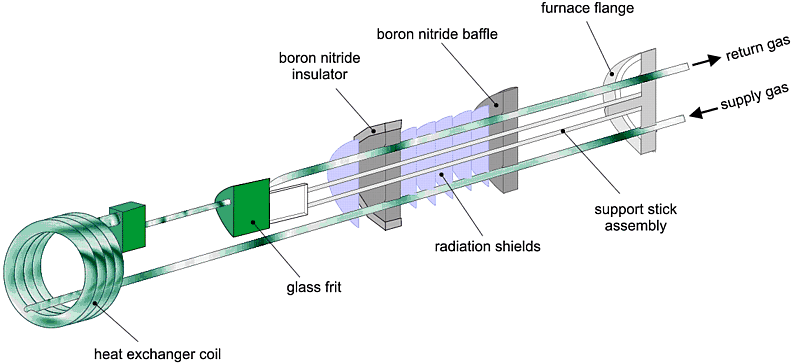
This cell has been used to study (by Erwin Lalik et al. of Birkbeck College and the ISIS neutron spallation source) the reduction in hydrogen at 550°C during an in-situ experiment lasting about 20 hours with a time resolution of 10 minutes; in total 123 neutron diffraction patterns were taken during the reaction, and these are shown below:
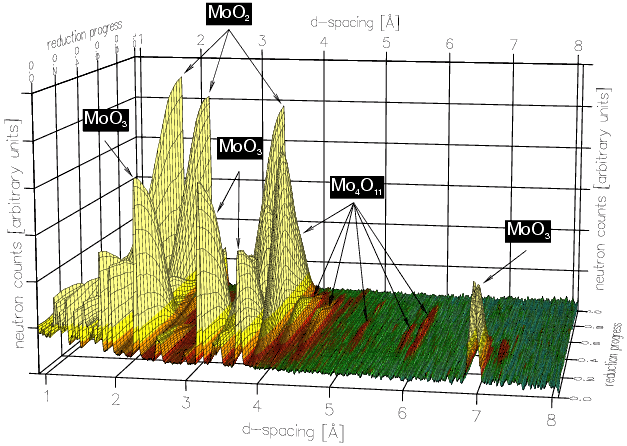
With these data sets one can perform multi-phase Rietveld refinement from which quantitative information, i.e. phase concentration versus time, can be obtained as shown in the plots below:
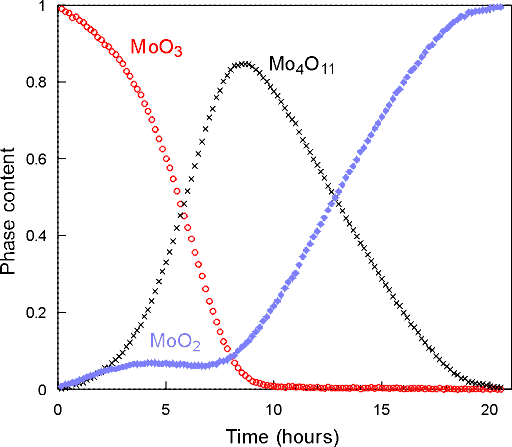
These results clearly confirm the formation of the orthorhombic Mo4O11. There are some other interesting features: (i) that the MoO2 grows initially with the Mo4O11 and (ii) the early plateau in the MoO2 formation. The overall kinetics of this sequence are quite complex, but the presence of the intermediate Mo4O11 phase discounts many of the previous mechanistic theories and gives a clearer idea of the structural re-arrangements involved in this reaction.
|
© Copyright 1997-2006.
Birkbeck College, University of London.
|
Author(s):
Paul Barnes Simon Jacques Erwin Lalik Martin Vickers |